Web-based flexible electronic data capture platform development SlimEDC
Patient prospective and retrospective studies and market research online.
About the product
Altamira is ISO certified software development company that brings more than 13 years of experience applying the world's leading practices. One of our standout products is SlimEDC, a web-based flexible electronic data capture (EDC) platform tailored for the pharmaceutical research sector. Retrospective and prospective studies, patient studies (ePRO) and market research projects ready to launch in no time, using the provided study editor.
Our Expertise
Web Development
Scope
Product Development
Vertical
Healthcare
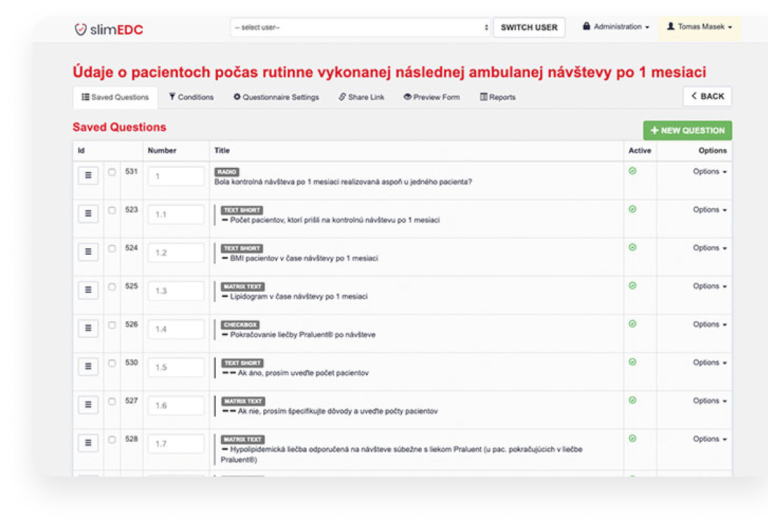
Key features
- User-friendly Study Editor
- Online Contracting System
- Live Interim Data Access
- SAS/SAP Compatibility
- Software as a Service
See what Altamira can do for you
01
The Challenge
The pharmaceutical industry constantly seeks ways to streamline research processes, especially in market research and clinical studies. Traditional methods of data capture are often cumbersome, expensive, time-consuming, and prone to errors. The market need was clear: a reliable, user-friendly, and compliant platform that could easily handle both simple and complex research studies.
So, we launched the development of SlimEDC tool to meet the emerging needs. Our objectives were:
- Simplify the process of electronic data capture for various types of studies.
- Ensure compliance with strict regulatory requirements (GDPR, ISO standards).
- Provide a platform that could be used by individuals with minimal IT experience.
During the development of the SlimEDC tool, Altamira faced several challenges, mainly the need to ensure compliance with strict regulatory standards and the EU environment and legislation. To address these issues, our team undertook multiple testing procedures and actively sought feedback from early users. Through this iterative process, the platform underwent continuous improvement to meet all requisite requirements and user expectations.
02
The Solution
SlimEDC is designed to transform the way data is captured in pharmaceutical research, providing a transparent, high-performing, and extremely user-friendly platform for various types of studies. The primary purpose of SlimEDC is to streamline the data collection process, ensuring accuracy, compliance, and ease of use for researchers, administrators, and respondents alike.
Altamira team used its expertise to design and implement SlimEDC, a lightweight yet powerful EDC platform with the following features:
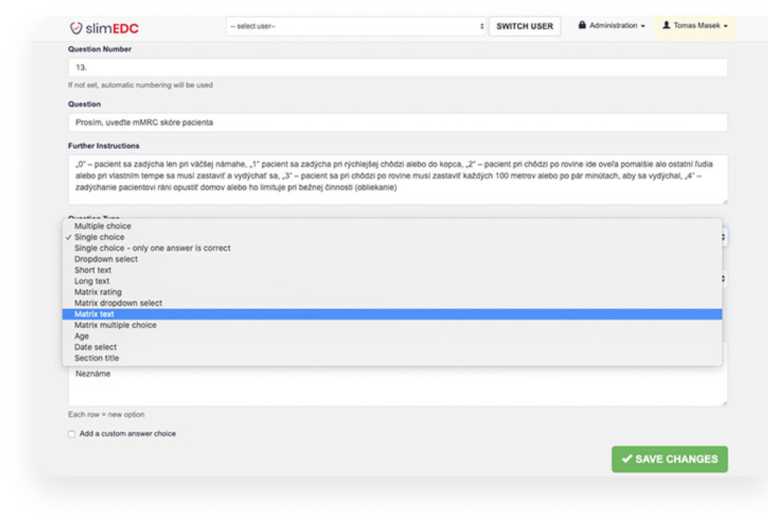
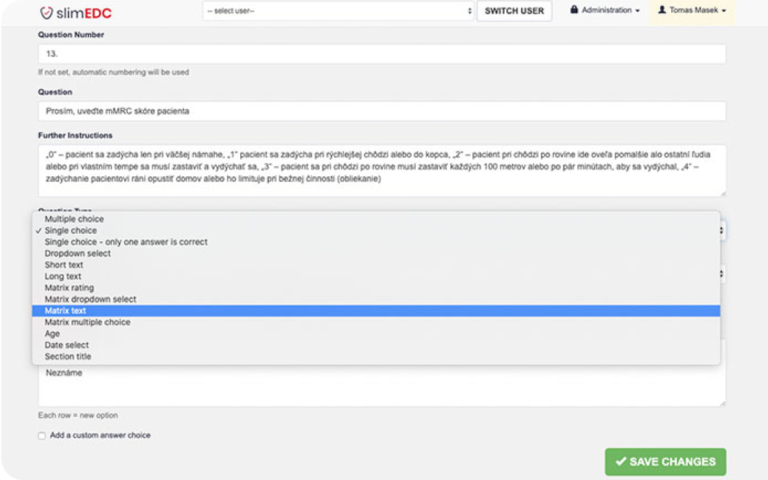
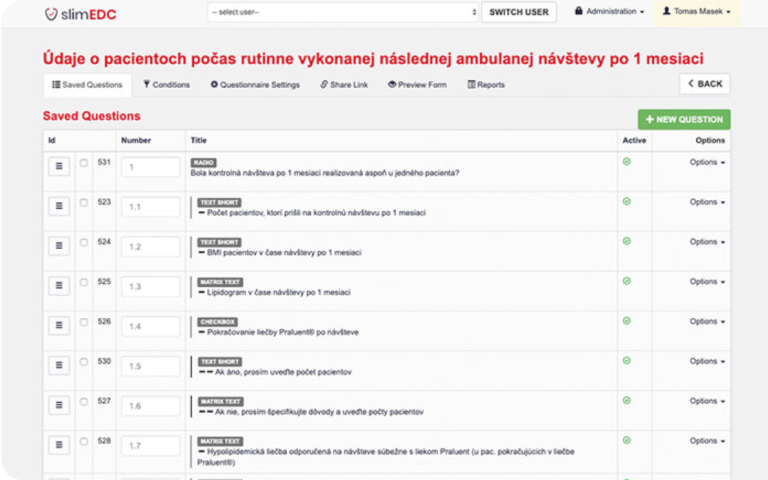
- User-friendly Study Editor: Enables the rapid creation of study questionnaires. The intuitive design ensures that even users with little technical background can create surveys within minutes.
- Online Contracting System: Allows for the electronic contracting of respondents, significantly reducing the time and effort needed to gather participants.
- Live Interim Data Access: Provides real-time access to partial study results, facilitating study management and quick adjustments.
- SAS/SAP Compatibility: Ensures that study results can be exported and integrated with major statistical tools like SAS and SAP.
- Software as a Service: SlimEDC is available as software fully managed by the research sponsor or as a complex service, where a professional SlimEDC team provides the entire process up to data evaluation.
Although SlimEDC is primarily a cloud-based platform, it can also be installed on-premise upon client request, offering flexibility to accommodate diverse needs. Furthermore, the platform adheres to all relevant EU regulations, ISO 9001, and 27001 standards, ensuring utmost data security and legal compliance.
Licensing model
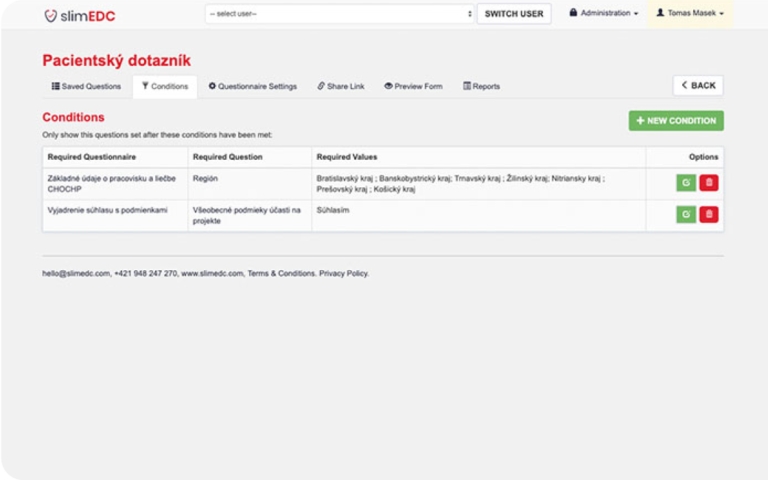
SlimEDC is designed with a flexible licensing model to accommodate various research needs. The licensing model includes:
- Subscription-based licensing: Clients can subscribe to SlimEDC on a monthly or annual basis, providing access to the platform and its support services. This model ensures that clients have the flexibility to scale their usage according to their project needs.
- One-time setup fee: For each new study, a one-time setup fee is applied, covering the initial configuration and customisation of the study. This fee is based on the complexity and duration of the setup process.
- Administrative fees: For projects involving respondent incentives, a per-respondent administrative fee is charged. This covers the processing of contracts and payouts, ensuring all legal requirements are met.
- Free trials and demos: SlimEDC offers free demos and trial periods to allow potential clients to experience the platform’s capabilities before committing to a subscription. Additionally, the platform offers complimentary setup assistance for new studies.
Strive for excellence with Altamira
Join our community of successful customers, whom we helped to build and grow their businesses.
03
The Result
Our team delivered an efficient tool enabling retrospective and prospective studies, patient studies (ePRO), and market research projects ready to launch in no time. As a result, SlimEDC transformed the way pharmaceutical research is conducted, delivering significant business value through its features:
- The user-friendly study editor and online contracting system significantly reduced the time needed to set up and manage studies. This efficiency allowed researchers to focus more on data analysis and less on administrative tasks.
- Real-time data access and compatibility with statistical tools ensured high-quality data management. The platform’s compliance with GDPR and ISO standards provided a secure and legally sound environment for data collection and processing.
- SlimEDC lowered operational costs by reducing the need for physical paperwork and manual processes. The flexibility of cloud and on-premise solutions also meant that organisations could choose the most cost-effective deployment option.
- Both respondents and administrators benefited from the intuitive interface, which required no additional training. This ease of use led to higher participation rates and more reliable data collection.
Through the development of SlimEDC, Altamira demonstrated its ability to deliver sophisticated, compliant, and user-friendly software products. Notably, SlimEDC is now used by prominent pharmaceutical companies such as Chiesi – one of the top 50 pharmaceutical companies in the world, Sandoz – the global leader in generic and biosimilar medicines, and Ipsen – a leading biopharmaceutical group, further validating its effectiveness and reliability in the industry. Our platform establishes a new standard for electronic data capture in pharmaceutical research.


